What does FLAVOUR mean?
Flavour is the sensation produced by a material taken in the mouth, perceived principally by the senses of taste and smell, and also by the general pain, tactile and temperature receptors in the mouth.
Flavour also denotes the sum of the characteristics of the material which produce that sensation.
Flavour is mainly composed of taste and odor. Texture has a very definite effect, smoothness, roughness, granularity, and viscousity can all h
What influences FLAVOUR?
ODOR
TEXTURE
~smoothness roughness granularity viscosity~
OTHERS
~hotness of spices coolness of menthol brothiness or fullness of certain amino acids metallic alkaline~
It is generally agreed that there are only FOUR BASIC or TRUE tastes; sweet, bitter, sour and salt. The sensitivity to taste is located in the taste buds of the tongue. There is a regional distribution of the four kinds of receptors to create areas of sensitivity.
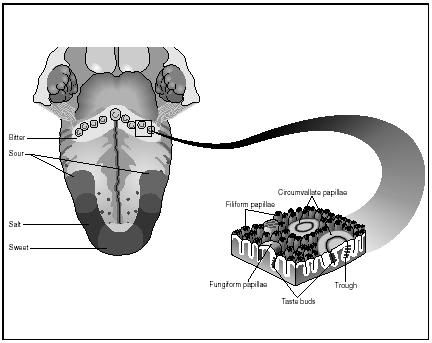
Chemical Structure and Taste
A first requirement for a substance to produce a taste is water-solubility. In general it can be said that all ACID substances are SOUR. Sodium chloride and other salts are salty, but as constituent atoms get bigger, bitter taste develops.
Minor changes in chemical structure may change the taste of a compound from sweet to bitter or tasteless.
The Effect of Substitutions in Saccharin on Sweetness
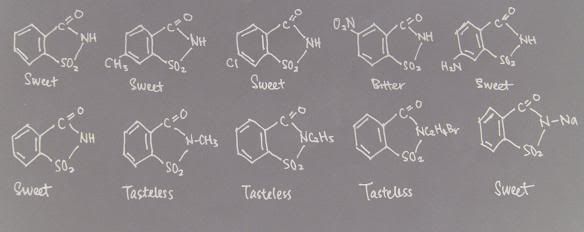
Saccharin is 500 times sweeter than sugar(pic above). Introduction of a methyl group or of chloride in para position reduces the sweetness by half. Placing a nitro group in the meta position makes the compound very bitter. Introduction of an amino group in the para position retains the sweetness. Substitutions at the imino group by methyl, ethyl or bromoethyl groups all result in tasteless compounds. However, introduction of sodium at this location yields sodium saccharin which is very sweet.

Oh! So tempting!
According to AH,B theory, all compounds which bring about a sweet taste response possess an electronegative atom A, such as oxygen or nitrogen. This atom also possesses a proton attached to it by a single covalent bond and, therefore, AH can represent a hydroxyl group, an imine or amine group, or a methine group.
The AH,B system present in sweet compounds is, according to Shallenberger, able to reach with a similar AH,B unit which exists at the taste bud receptor site through the formation of simultaneous hydrogen bonds. The relatively strong nature of such bonds could explain why the senses of sweetness is a lingering sensation.

*cringes*
Although it is generally recognized that the sour taste is a property of the hydrogen ion, there is no simple relationship between sour taste and acid concentration.

MSG aint good! nuh-uh
The salty taste is best exhibited by sodium chloride. It is sometimes claimed that the taste of salt by itself is unpleasant, and that the main purpose of salt as a food component is to act as a flavour enhancer or flavour potentiator. The taste of salts is dependent on the nature of both cation and anion.

Oh gourd, they are friggin' bitter..
The bitter taste is widely distributed and can be attributed to a great variety of organic and inorganic compounds. Although bitter taste by itself is usually considered unpleasant, it is a common component of the taste of many foods usually in combination with sweet and sour.