ok ok..the real reason why I chose to write about this is that my mom has been making enzyme since last month after she read a news article about this. Since it's environmental friendly and pretty useful, she was quite supportive with that idea. I'm gonna point out some interesting chemical facts about enzymes and ways to make them. So, here goes:
ENZYMES
enzymes
This is what it says in the video:
"Enzymes enable molecules called substrates to undergo a chemical change to form new substances called products. Each enzyme acts on a specific molecule or a set of molecules called substrates. Each substrate fits into an area of the enzyme called the active site. This fitting together is often compared to a lock and key mechanism. However, the enzyme changes shape a little to fit with the substrate. In the enzyme-substrate complex, the enzyme holds the substrate or substrates in a position where a reaction can occur easily. After reaction, the enzyme releases the products and could go on to carry out the same reaction again and again."
All enzymes are proteins and can be classified as either:
1.Simple proteins whose catalytic activities rely only on the enzyme's polypeptidyl structure
2.Conjugated proteins which require a non-protein component(s) for activity
The non-protein components of enzymes, called cofactors, include metal ions and organic biomolecules(coenzymes) like B-vitamins. If a cofactor is tightly bound to an enzyme, it is called a prosthetic group.
An enzyme is classified by the type of reaction it catalyzes, and the six major classes of enzymes are:
oxido-reductase
transferases
hydrolases
lyases
isomerases
ligases
In an enzyme-catalyzed reaction, increasing the substrate concentration increases the reaction rate(v). However, after the active sites of the enzyme molecules are saturated with substrate, the reaction rate becomes independent of substrate concentration (zero-order reaction).
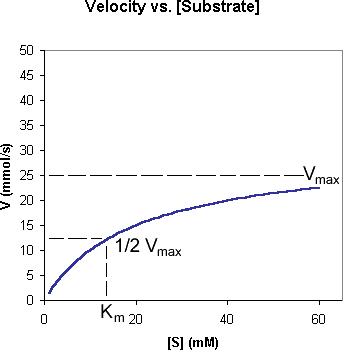
The maximal velocity rate theoretically attainable under these conditions is called Vmax. The substrate concentrationat which the reaction rate is half of that of Vmax is called the Km(Michaelis constant). Each enzyme displays characteristic Km for its substrate(s). The relationship among substrate concentration,v,Vmax,Km, and the initial reaction rate of an enzyme reaction is mathematically expressed in the Michaelis-Menten equation.
The Km and the Vmax values of an enzymatic reaction are often determined by plotting the reciprocals of [S] and v values(Lineweaver-Burk plot). Ideally, the Lineweaver-Burk plot yields a straight line with a slope equals to Km/Vmax, and the 1/v and 1/S intercepts are equal to 1/Vmax and -1/Km respectively.
Pretty confusing but the graph makes my life and yours(I hope) easier:
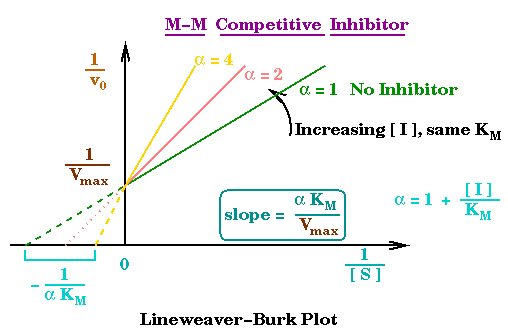
Three general types of inhibition of enzymatic activity are called competitive, non-competitive, and
ok ok..enough of facts.
How to make garbage enzymes?
1. Use the ratio of 1:3:10 (sugar:vegetable/fruit dregs:water).
2. For the sugar component, use jaggery or molasses.
3. For a fresh-smelling enzyme, add orange, or lemon peels, or pandan leaves. (Vegetable waste will give an unpleasant smell).
4. Use an air-tight plastic container to allow expansion. Do not use glass or metal containers. Place the container in a cool, dry, and well-ventilated area. Avoid direct sunlight. Open the container daily in the first month to release gases.
5. If you have not gathered enough kitchen waste, you may fill the container gradually. The three-month fermentation process starts with your last batch of kitchen waste. Push the floating garbage down once in a while.
6. The enzyme liquid should be dark brown in colour. It it is blackish, add in the same amount of sugar to start the fermentation process again.
7. Ignore any white, black or brown layer that forms on the surface of the mixture. This is just yeast. If the container is not airtight, flies or maggots may appear. Leave them and the chemical reaction will resolve them naturally.
8. After 3 months, filter the fermented liquid through a sieve. Use the residues in your new batch of enzyme or as fertiliser.
9. The enzyme will never expire and must be diluted with water for use.
Here's a picture of the enzyme my mom made:

and another one..

References:
http://www.chemeddl.org/collections/netorials/biomolecules/enzymes/enzyme4.htm
http://dept.physics.upenn.edu/courses/gladney/mathphys/subsection4_1_7.html
7 April 2009, The Star newspaper